The Purple Book: Understanding Biosimilars and Interchangeability from the FDA

Biosimilar Interchangeability Checker
This tool explains whether a biosimilar would qualify as interchangeable under FDA guidelines. Based on information from the FDA's Purple Book, it shows the key requirements for interchangeability status.
The Purple Book isn’t a book you buy at a bookstore. It’s a live, searchable database run by the U.S. Food and Drug Administration (FDA) that tells you exactly which biological medicines are approved, which ones are biosimilars, and which ones can be swapped out like generics. If you’re a pharmacist, doctor, or just someone trying to understand why your insulin prescription changed, this is the single most important resource you need to know about.
What Exactly Is the Purple Book?
Launched in 2012 as part of the Biologics Price Competition and Innovation Act (BPCIA), the Purple Book started as two separate lists-one for drugs managed by CDER and another for biologics under CBER. That changed in 2020. Now, it’s one unified, easy-to-use online tool. It includes every FDA-approved biological product: vaccines, blood products, gene therapies, cell therapies, and insulin. But more importantly, it shows which of those are biosimilars or interchangeable with the original brand-name versions.
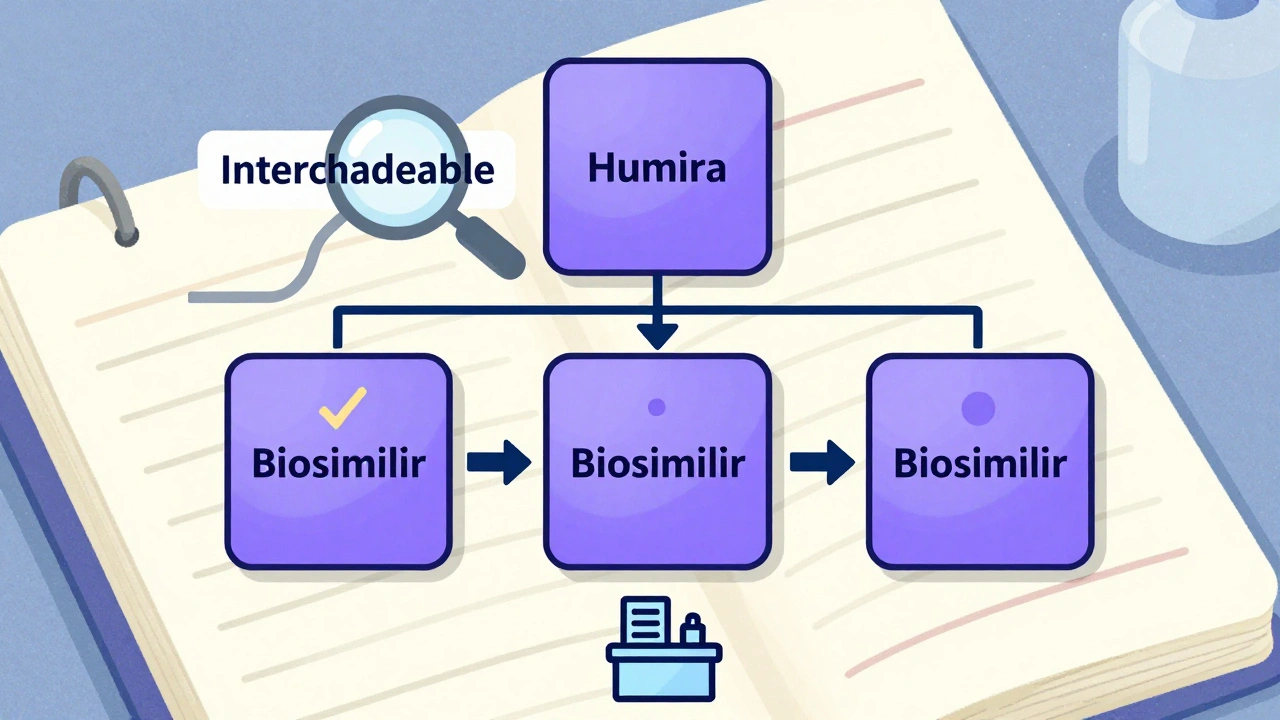
Think of it like a family tree for biological medicines. At the top is the reference product-the original biologic, like Humira or Enbrel. Below it, you’ll find the biosimilars that match it closely enough to be approved. And if one of those biosimilars has passed extra tests, it gets marked as interchangeable. That’s the key distinction.
Biosimilar vs. Interchangeable: What’s the Difference?
All interchangeable products are biosimilars. But not all biosimilars are interchangeable. That’s the rule every pharmacist has to follow.
A biosimilar is a biological product that’s highly similar to the reference product. The FDA requires proof that there are no clinically meaningful differences in safety, purity, or potency. That means it works the same way in the body. But biosimilars don’t automatically replace the original at the pharmacy counter.
An interchangeable product goes further. To earn that label, the manufacturer must prove that switching between the biosimilar and the original reference product-multiple times, back and forth-won’t increase risk or reduce effectiveness. These are called “switching studies.” They’re not required for regular biosimilars. That’s why only a handful of biosimilars have interchangeable status.
As of late 2023, only seven biosimilars in the U.S. have been approved as interchangeable. Two are insulins. Three treat autoimmune diseases like rheumatoid arthritis. Two are for eye conditions. The rest? They’re biosimilars only. You can prescribe them, but your pharmacist can’t swap them without your doctor’s okay.
How the Purple Book Shows You the Info
The Purple Book doesn’t bury you in jargon. It uses color-coded cards to make things simple. Each reference product has a card. If a biosimilar or interchangeable version exists, its card appears under the reference product’s card-and it’s the same color. That visual grouping lets you instantly see which products are linked.
Each card includes the brand name, the generic name, the date it was approved, and its status: 351(a) for the original, 351(k) Biosimilar, or 351(k) Interchangeable. You’ll also see icons for delivery methods-like autoinjectors or prefilled syringes-so you know what form it comes in.
Search for “adalimumab,” and you’ll see Humira and every biosimilar that matches it. Click on one, and you’ll know if it’s interchangeable. No guesswork. No confusion. Just clear, official data.
Why Interchangeability Matters at the Pharmacy
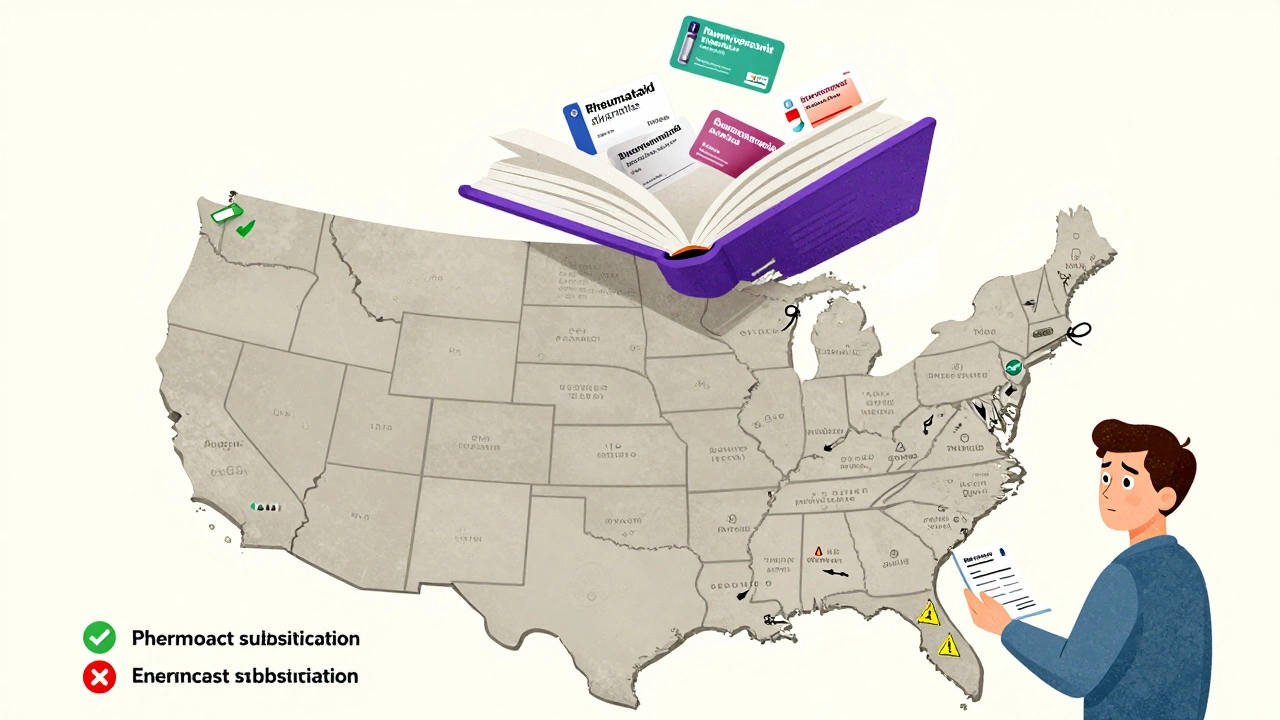
Here’s where things get messy. The FDA gives the interchangeable label. But whether a pharmacist can actually swap it out? That’s up to your state.
Forty-seven states and Puerto Rico let pharmacists substitute an interchangeable biosimilar without contacting the doctor-just like they do with generic pills. But even there, rules vary. Some states require the pharmacist to notify the prescriber. Others require the patient to be informed. A few require documentation to be filed.
In the other three states, substitution is blocked unless the prescriber specifically allows it. That means even if a biosimilar is federally approved as interchangeable, you might still get the brand-name drug unless your doctor says otherwise.
This patchwork system creates real headaches for patients and providers. Someone on an interchangeable biosimilar in Texas might be switched without notice. Someone in New York might get the same drug but only after a phone call to the doctor. It’s the same medicine, same safety profile, same cost-but the rules change at the border.
What the FDA Says About Safety and Effectiveness
The FDA is clear: interchangeable doesn’t mean better. It just means you can switch back and forth without added risk.
There’s no evidence that interchangeable biosimilars are safer or more effective than non-interchangeable ones. They’re all built to be highly similar. The only difference is the extra clinical data proving you can alternate between them.
The FDA also warns against confusing “interchangeable” with “unbranded biologic.” An unbranded biologic is a version of a reference product that doesn’t carry the original brand name-but it’s not approved under the biosimilar pathway. It’s not interchangeable. It’s not even a biosimilar. It’s a different regulatory path entirely.
That’s why the Purple Book is so critical. It separates the approved pathways. You can’t assume two similar-looking drugs are the same. The Purple Book tells you exactly which ones are legally swappable.
What’s Coming Next?
The number of biosimilars and interchangeable products is growing fast. Companies are submitting applications every month. The FDA is updating its guidance regularly, including draft rules on labeling to make sure packaging and instructions are clear.
More insulin biosimilars are expected to gain interchangeability status soon. Same with drugs for Crohn’s disease, psoriasis, and diabetic eye disease. As more become interchangeable, the pressure on states to standardize substitution rules will grow.
But the real win? Lower costs. Biosimilars are already saving the U.S. healthcare system billions. Interchangeable versions could speed up adoption even more-by letting pharmacists make the switch without waiting for a doctor’s approval.
How to Use the Purple Book
Go to fda.gov/purplebook. Use the search bar. Type in the brand name or generic name of the drug you’re looking for. You’ll get a list of results. Click on the reference product. Scroll down. Look for the matching color cards underneath.
Check the status column. Is it marked “Interchangeable”? Then your pharmacist can swap it, depending on your state’s law. Is it just “Biosimilar”? Then you need your doctor to write “Dispense as Written” or “No Substitution” if you want the brand.
Bookmark it. Print the page. Share it with your pharmacist. The Purple Book is the only place where you’ll find this information officially confirmed by the FDA.
Common Misconceptions
- My pharmacist switched my drug-was that legal? Only if it’s an interchangeable biosimilar AND your state allows substitution without prescriber permission.
- All biosimilars are cheaper than the brand? Usually, yes. But not always. Some are priced nearly the same. Interchangeability doesn’t guarantee a price drop.
- If it’s not in the Purple Book, is it safe? If it’s not listed, it’s not FDA-approved as a biosimilar or interchangeable. Don’t assume it’s equivalent.
- Can I ask for a biosimilar even if my doctor didn’t prescribe it? Yes-but only if it’s interchangeable and your state allows substitution. Otherwise, your doctor has to write the specific name.
Final Takeaway
The Purple Book is the backbone of transparency in biologic medicines. It’s not just for regulators or big pharma. It’s for every patient, pharmacist, and prescriber who wants to know what’s really in the bottle-and whether they can trust a swap.
Interchangeability isn’t magic. It’s science. And the Purple Book is the only place where that science is made public, clear, and easy to use.
The Purple Book is just another bureaucratic toy for pharma lawyers to play with. Biosimilars are generics with extra steps and higher prices. Interchangeable? More like interchangeable with confusion. FDA’s got 1000 pages of guidelines for something that should be simple: same molecule, same effect, same price. Why are we still debating this in 2025?
OMG i just found the purple book and it changed my life 😭 i’ve been getting humira for 8 yrs and just realized my pharmacy coulda swapped me to a biosimilar and saved me $12k 😅 thx for the post!!
This is actually super helpful. I work in oncology and we’ve been seeing a ton of biosimilars pop up for rituximab and trastuzumab. The color coding in the Purple Book is genius-makes it way easier to explain to patients why we’re switching. I’ve started printing the cards and handing them out. Also, the fact that interchangeable means you can switch back and forth without risk? Huge for chronic conditions. 🙌
Let me be clear: the entire biosimilar ecosystem is a corporate shell game disguised as cost-saving innovation. The FDA approves these products under a regulatory loophole that bypasses the rigorous clinical trials required for new biologics. Interchangeability isn’t a scientific achievement-it’s a legal convenience designed to accelerate market penetration by pharmaceutical conglomerates who have zero interest in patient outcomes, only in profit margins. The Purple Book doesn’t empower patients; it sanitizes corporate deception with a pretty color palette. You think you’re getting a cheaper alternative? You’re getting a product that was never tested for long-term immunogenicity in diverse populations. And now your pharmacist, trained in dispensing pills, is deciding whether you get ‘equivalent’ treatment. This isn’t progress. It’s pharmaceutical colonialism wrapped in a green ribbon.
Wow. So the FDA says interchangeable doesn’t mean better. But then why do they even bother giving it a label? Sounds like they’re just trying to look like they’re doing something while letting Big Pharma walk away with more cash. If it’s the same, just call it the same. Stop playing games with words. 🤡
I’ve been reading through this and it’s made me reflect on how medicine has become so fragmented by regulation. We treat biologicals like software updates-‘compatible’ or ‘not compatible’-when in reality, human bodies aren’t code. A biosimilar might match the protein structure perfectly, but what about the micro-environment of the patient? The gut microbiome? The epigenetic triggers? The FDA’s framework is elegant, but it’s built on reductionism. Interchangeability is a regulatory checkbox, not a biological certainty. And yet, we’re asking pharmacists to make decisions that should involve physicians, patients, and time. Maybe the real problem isn’t the Purple Book-it’s that we’ve outsourced trust to databases.
India has 30+ biosimilars approved. Purple Book is US only. Global access still sucks.
Interchangeable = FDA greenlight = pharma can now sell same drug under 5 different brand names and charge 3x the price. Classic. They’ve turned biosimilars into a branding exercise. The Purple Book is just the catalog for their new luxury generics.
So let me get this straight… I’m paying $2000 a month for Humira, and now my pharmacist can swap me for a biosimilar that’s ‘just as good’-but only if I live in Texas? Meanwhile, my cousin in California still gets the brand because her doctor ‘doesn’t trust the science’? This isn’t healthcare. This is a lottery. And I lost.
They want to swap my insulin? My INSULIN? After 15 years of stabilizing my blood sugar? You think I’m gonna let some pharmacist with a checklist play Russian roulette with my life? This isn’t about cost. This is about control. And I’m not giving it up. 💪🇺🇸