Immunosuppressants: Cyclosporine and Tacrolimus Generic Issues
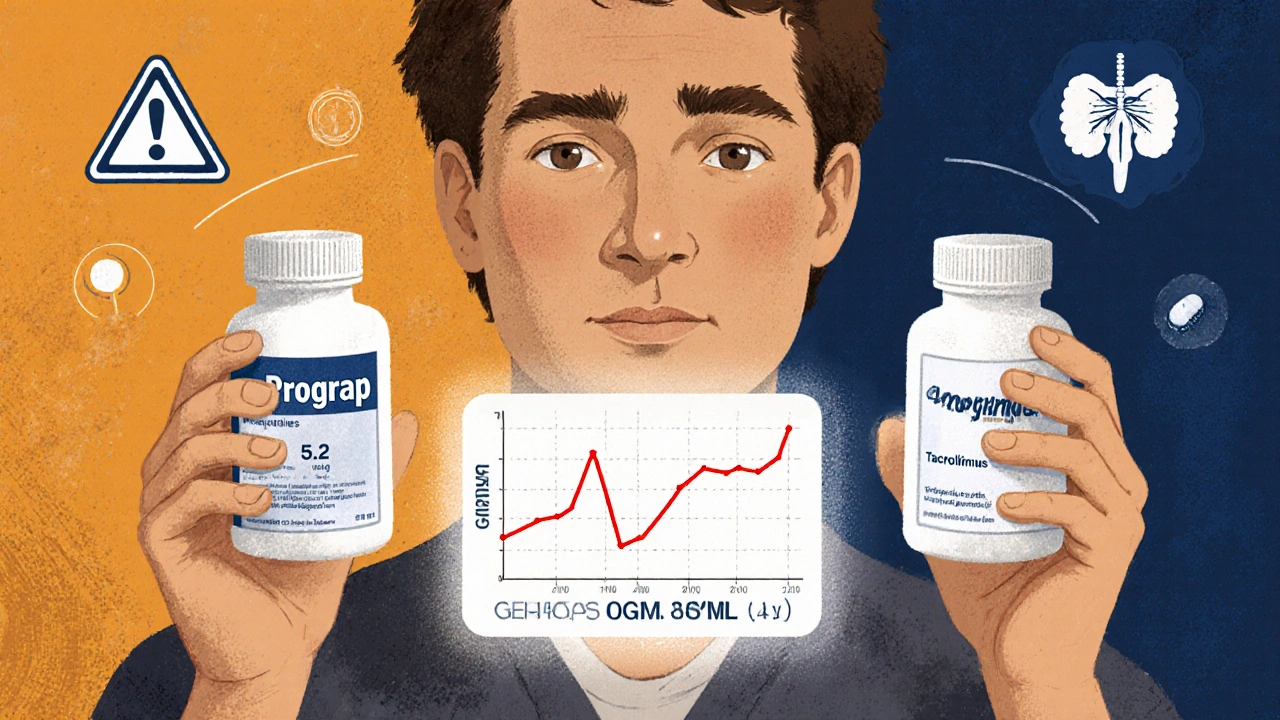
When you’ve had a transplant, your life changes in ways most people never think about. One of the biggest daily concerns isn’t just recovery-it’s making sure your immunosuppressant meds work exactly right. Two drugs, cyclosporine and tacrolimus, are the backbone of most transplant regimens. They keep your immune system from attacking the new organ. But here’s the problem: when your insurance forces you to switch from brand-name to a generic version, things can go wrong-sometimes dangerously so.
Why These Drugs Are So Tricky
Cyclosporine and tacrolimus are both calcineurin inhibitors. That means they block a key part of your immune system’s activation. But they’re not the same. Tacrolimus works at 20 to 100 times lower doses than cyclosporine. You might take 5 mg of tacrolimus twice a day, but 150 mg of cyclosporine for the same effect. That difference in potency is why even tiny changes in how your body absorbs the drug can throw everything off. Both drugs have a narrow therapeutic index. That’s medical jargon for: the difference between a dose that works and one that’s toxic is very small. For tacrolimus, the safe range is 5-15 ng/mL in the blood during the first few months after transplant. Go below 5, and your body might reject the organ. Go above 15, and you risk kidney damage, seizures, or even death. Cyclosporine’s range is wider-100-200 ng/mL-but still tight enough that a 10% change in absorption can mean trouble.Generic Versions Are Everywhere-But Not All Are Equal
In 2025, over 92% of tacrolimus and cyclosporine prescriptions in the U.S. are generic. That’s because they cost a fraction of the brand names. Prograf (tacrolimus) used to run $1,200-$1,500 a month. Generic versions now cost $300-$500. Neoral (cyclosporine) dropped from $800-$1,000 to $150-$300. That’s huge savings. But cost isn’t the whole story. There are 14 FDA-approved generic tacrolimus products from 8 different manufacturers. For cyclosporine, there are 11 generics from 7 companies. Each one uses slightly different fillers, coatings, or oil-based carriers. That affects how fast or how well your body absorbs the drug. A 2023 study in Pharmacoepidemiology and Drug Safety found that only 42% of generic manufacturers provide detailed bioequivalence data to doctors. Most don’t. So you’re switching to a version your doctor never tested on your body.Real People, Real Problems
Reddit threads and transplant forums are full of stories like this:- One user switched from Prograf to a generic tacrolimus and saw their blood levels drop from 8.5 to 5.2 in two weeks. They had a mild rejection episode and ended up in the hospital.
- Another person tried a different generic cyclosporine and couldn’t get stable levels for months. Their nephrologist refused to let them switch again.
- A third person saved $900 a month with a generic and had no issues-stable levels for 18 months.
Why Switching Between Generics Is Risky
Here’s where it gets worse. Some pharmacies switch the generic brand every time you refill-just because the cheapest one is in stock. You might get one generic in January, another in February, and a third in March. Each one has a different absorption profile. That’s not just inconvenient. It’s dangerous. The European Medicines Agency warned in 2024 that switching between generic tacrolimus products without monitoring can cause subtherapeutic or toxic levels. The FDA’s approval standards require generics to be within 80-125% of the brand’s absorption-but that’s a huge range. Two generics can both be “bioequivalent” to the brand, but not to each other. That’s why transplant centers now recommend staying on the same generic brand once you’ve stabilized on it.What Works: Real Strategies for Safety
If you’re on a generic immunosuppressant, here’s what you need to do:- Stick with one generic brand. If your levels are stable on Generic A, don’t switch to Generic B unless your doctor says so. Ask your pharmacist which brand you’re getting each time.
- Monitor your levels closely after any switch. Most transplant centers check blood levels weekly for the first month after switching. Don’t skip these tests.
- Avoid grapefruit and Seville oranges. These interfere with how your liver breaks down both drugs, making levels unpredictable.
- Take your dose at the same time every day. A 2-hour window is okay. A 6-hour swing? That can cause spikes or drops.
- Know your target range. Ask your doctor: “What’s my ideal blood level right now?” Write it down. If your level is outside that range, don’t wait-call your transplant team.

What’s Changing in 2025
There’s some good news. In late 2023, the FDA approved a new extended-release version of tacrolimus called LCP-tacrolimus. It releases the drug slowly, reducing the peaks and valleys in blood levels. That means fewer fluctuations-and less risk when switching generics. Also, in early 2024, the European Medicines Agency updated its rules. Now, generic manufacturers must prove bioequivalence using actual transplant patients-not just healthy volunteers. That’s a big step. It means future generics will be tested under real-world conditions. Another emerging solution? Genetic testing. About 20% of people have a gene variant (CYP3A5) that makes them metabolize tacrolimus faster. If you’re one of them, you need a higher dose. A 2023 JAMA Internal Medicine study showed that dosing based on CYP3A5 genotype cuts the time to reach stable levels by 63%. More transplant centers are starting to offer this test.The Bottom Line
Generic immunosuppressants save lives-by making these drugs affordable. But they’re not interchangeable. They’re not all the same. You can’t treat them like ibuprofen or statins. If you’re on cyclosporine or tacrolimus, your medication isn’t just a pill. It’s a precision tool. And like any precision tool, it needs careful handling. Don’t let cost savings come at the cost of your transplant. Stay informed. Ask questions. Track your levels. And never, ever switch brands without talking to your transplant team first.Can I safely switch between different generic versions of tacrolimus?
No-not without close monitoring. Even though all generics are FDA-approved, they can have different absorption rates. Switching between brands can cause your blood levels to drop too low (risking rejection) or spike too high (risking toxicity). Most transplant centers require weekly blood tests for at least four weeks after any switch.
Why is tacrolimus preferred over cyclosporine?
Tacrolimus is more effective at preventing acute rejection and leads to better long-term kidney function. Studies show rejection rates are nearly half with tacrolimus compared to cyclosporine. However, tacrolimus carries a higher risk of post-transplant diabetes and neurological side effects like tremors. For most patients, the benefits outweigh the risks.
How do I know if my generic immunosuppressant is working?
You need regular blood tests to measure drug levels. For tacrolimus, aim for 5-15 ng/mL in the first 6 months after transplant. For cyclosporine, it’s 100-200 ng/mL. If your levels are outside this range, or if you feel new symptoms like fatigue, nausea, or tingling, contact your transplant team immediately. Don’t wait.
Does insurance force me to use generics?
Yes, in most cases. Medicare Part D and many private insurers require generics unless your doctor files a medical exception. If your doctor believes a brand-name drug is medically necessary, they can appeal. But you’ll need documentation-like previous rejection episodes or unstable levels on generics-to support the request.
Are there any foods or supplements I should avoid?
Yes. Grapefruit, Seville oranges, pomelos, and starfruit can dramatically increase drug levels. St. John’s wort, echinacea, and garlic supplements can lower them. Even some antibiotics and antifungals interact. Always check with your pharmacist before taking anything new-prescription, over-the-counter, or herbal.
What should I do if I miss a dose?
If you miss a dose, take it as soon as you remember-if it’s within 4 hours of your scheduled time. If it’s more than 4 hours late, skip it and take your next dose at the regular time. Never double up. Missing doses increases rejection risk. If you miss more than one, contact your transplant center immediately.
Can I switch back to the brand-name drug if generics cause problems?
Yes, but it’s not easy. You’ll need your doctor to file a prior authorization with your insurer, showing that generics caused instability, rejection, or toxicity. Keep detailed records of your blood levels, symptoms, and dates of switches. Many patients succeed with this process, especially if they’ve had a serious event linked to a generic switch.
This is one of those topics that doesn’t get enough attention. I’ve been on tacrolimus for 7 years. Switching generics once nearly cost me my kidney. Never again.
From a pharmacokinetic standpoint, the inter-individual variability in CYP3A4/5 metabolism makes generic substitution particularly perilous for calcineurin inhibitors. Bioequivalence in healthy volunteers doesn’t translate to transplant recipients.
So glad you laid this out so clearly. If you're on these meds, track your levels like your life depends on it-because it does. And don’t be shy about asking your pharmacist which generic you’re getting. Knowledge is power.
Big Pharma and the FDA are in bed together. They don’t care if you reject your organ as long as the stock price goes up. 🤡
I had the opposite experience-switched to a generic cyclosporine and never had an issue. My levels stayed rock solid for over two years. But I also took every test, every checkup, and never skipped a dose. Maybe it’s not the generic-it’s how you manage it.
As someone from India where generics are the only option for most, I get how vital affordability is. But this post reminds me: even in places with limited access, we need to push for better quality control. Lives depend on it.
Wait-so two generics can both be "bioequivalent" to the brand… but not to each other?! That’s not bioequivalence-that’s a statistical loophole. The FDA’s 80-125% range is basically saying, "We’re okay if your drug works 20% worse or 25% better." That’s not medicine. That’s gambling. And we’re the ones betting our organs.
My brother had a liver transplant. He switched generics, got dizzy and nauseous for weeks. They thought it was stress. Turned out his tac levels dropped to 3.2. He almost lost it. Now he won’t touch anything but the brand. Insurance hates it. He doesn’t care.
It is imperative that patients maintain consistent medication adherence and undergo regular therapeutic drug monitoring following any generic substitution. The clinical implications of fluctuating serum concentrations are well documented in the literature and should not be underestimated.
There’s a deeper truth here: we treat transplants like a one-time fix. But the real work begins after the surgery. You’re not just living with a new organ-you’re living with a constant, invisible negotiation between your body and chemistry. These drugs aren’t pills. They’re lifelines. And the system treats them like commodity goods. That’s not just flawed. It’s cruel. I’ve seen people lose everything because someone in a warehouse picked the cheapest bottle. We need to stop pretending this is just about cost. It’s about dignity.